李湘女儿王诗龄去英国留学啦,随即博耐顿女校再次出圈,这所学校是英国顶尖的私立女校,它曾是英国王室安妮公主、赌王女儿何超欣等贵族、名人送女儿去这所学校,该校的学术成绩也十分优异,每年有20%的学生升入牛津、剑桥大学,可是,入读这所私校要经过笔试,下面,小编就为大家带来了博耐顿女校Benenden School year12化学入学考试笔试题库,希望对大家有所帮助:

化学
时间:1小时30分钟
考生须知:
•在上面的方框中填写你的姓名、学校和日期
•使用黑色墨水或圆珠笔。如果你改变了对一个答案的想法,请在它之间画上一条线,然后写下你的新答案(请不要使用Tipex或涂改液)。
•回答所有问题
•在空白处回答问题
•展示所有计算步骤,并记得包含单位
•你可以使用计算器
•使用提供的元素周期表
•括号内标明了每个问题的分数
这篇论文的总分是100分
Questions
Q1.

(b) Acids also react with metal carbonates.
The word equation for the reaction of copper carbonate with dilute nitric acid is

(i) State two things you would see when solid copper carbonate reacts with dilute nitric acid.
..............................................................................................................................................
..............................................................................................................................................
..............................................................................................................................................
(ii) Write the balanced equation for the reaction of copper carbonate with dilute nitric acid.
..............................................................................................................................................
(c) Two gases can be produced by the electrolysis of water, under suitable conditions.
(i) Explain what is meant by electrolysis.
..............................................................................................................................................
..............................................................................................................................................
..............................................................................................................................................
(ii) One of the gases is hydrogen. Describe a test to show the gas is hydrogen.
..............................................................................................................................................
Q2.
The names and formulae of the first three alcohols in the homologous series of alcohols are given in the table.
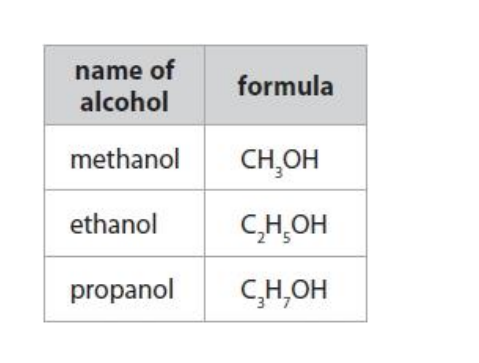
(i) Pentanol is another member of the alcohol homologous series.
A molecule of pentanol contains five carbon atoms.
Predict the formula of a molecule of pentanol.
...........................................................
(ii) Draw the structure of a molecule of ethanol, C2H5OH, showing all the bonds.
Q3.
Lithium, sodium and potassium are metals in group 1 of the periodic table.
They are good conductors of heat and electricity.
The freshly-cut metals are shiny.
(a) (i) Give another physical property of all three of these metals.
(ii) Explain, in terms of electrons in their atoms, why lithium, sodium and potassium are in group 1 of the periodic table.
(b) A small piece of potassium is added to water.
(i) Describe what you would see in this reaction.

(c) There is an increase in reactivity of these group 1 metals from lithium to potassium. Explain this increase in reactivity.
Q4.
(a) The table shows the number of electrons, neutrons and protons in particles P, Q, R, S, T and V.
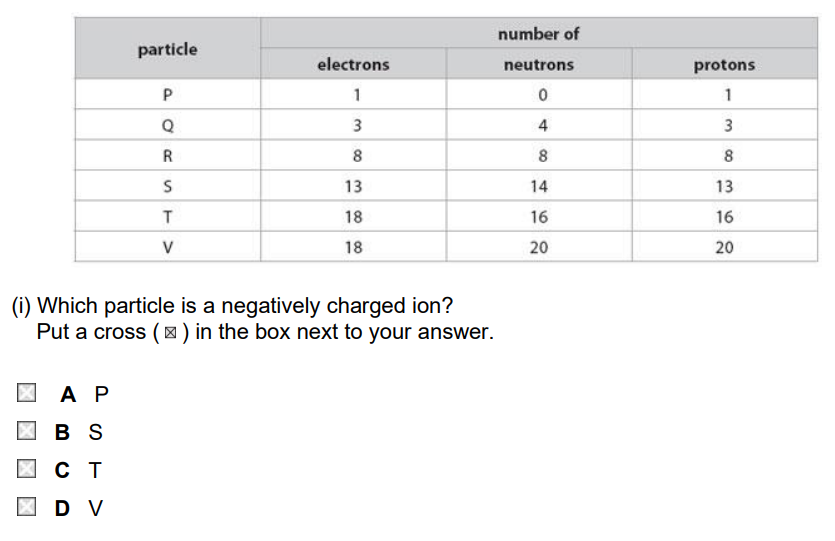

(b) Each element has an atomic number.
(i) State what is meant by atomic number.
..............................................................................................................................................
..............................................................................................................................................
(ii) The atomic number of boron is 5.
Boron exists as two isotopes boron-10 and boron-11.
Use this information to explain why boron-10 and boron-11 are isotopes.
..............................................................................................................................................
..............................................................................................................................................
(c) (i) Explain what is meant by the term relative atomic mass.
..............................................................................................................................................
..............................................................................................................................................
(ii) A sample of boron contains
• 19.7% of boron-10.
• 80.3% of boron-11.
Use this information to calculate the relative atomic mass of boron.
..............................................................................................................................................
..............................................................................................................................................
Q5.
Marble chips react with dilute hydrochloric acid.
Marble is a form of calcium carbonate.
(i)Complete the balanced equation for this reaction.
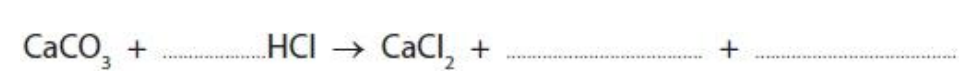
(ii) Explain how using smaller sized marble chips affects the rate of this reaction, when all the other conditions remain the same.
.............................................................................................................................................
.............................................................................................................................................
(iii) Explain, in terms of collisions between particles, how increasing the concentration of the hydrochloric acid affects the rate of this reaction, when all the other conditions remain the same.
.............................................................................................................................................
.............................................................................................................................................
Q6.
Copper hydroxide, copper oxide and copper sulfide are three compounds of copper.
In an analysis of copper sulfide, 12.7 g of copper was found to be combined with 3.2 g of sulfur.
Calculate the empirical formula of the copper sulfide.
Show your working.
(relative atomic masses: Cu = 63.5, S = 32).
.............................................................................................................................................
Q7.
The table shows some properties of six compounds.
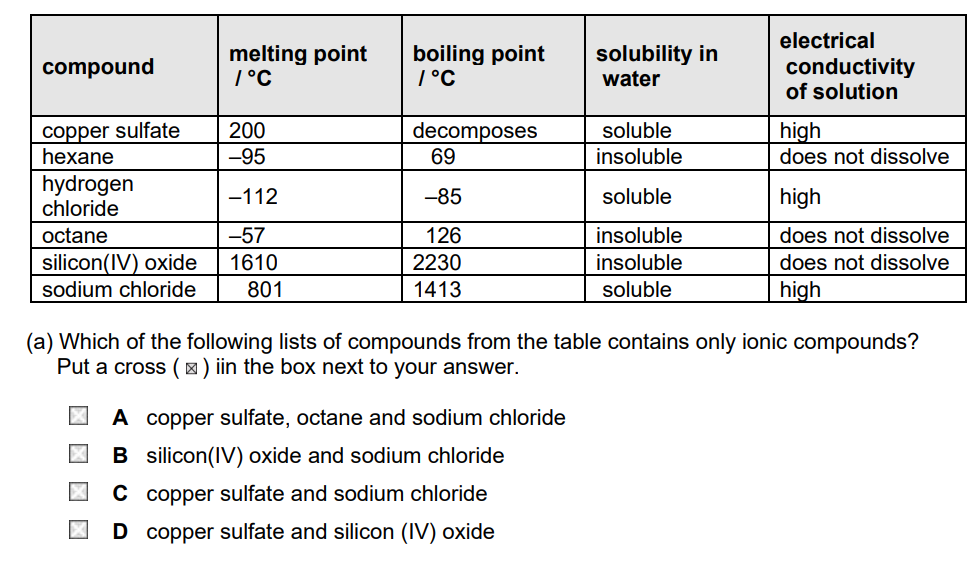
(b) Two of the compounds in the table produce a colour in a flame test.
Give the name of one of these compounds and the colour it produces in the flame test.
compound . . . . . . . . . . . . . . . . . . . .
colour . . . . . . . . . . . . . . . . . . . .
(c) Hexane is a covalent compound containing simple molecules.
It has a low boiling point.
(i) Explain why it has a low boiling point.
(ii) Hexane and water are immiscible.
Describe how separate samples of hexane and water can be obtained from a mixture of Hexane and water.
(d) Draw a dot and cross diagram of a molecule of hydrogen chloride.
Show outer electrons only.
The table shows some properties of diamond and graphite.
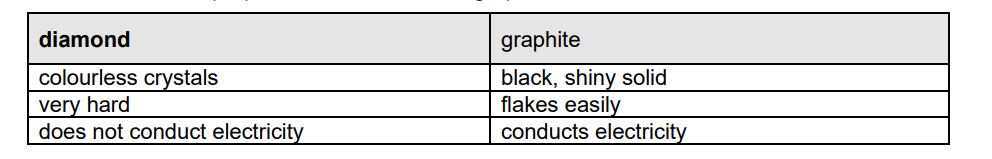
(i) Suggest why diamond and graphite might be expected to have similar properties.
..............................................................................................................................................
..............................................................................................................................................
(ii) By referring to its structure, explain why diamond is very hard.
..............................................................................................................................................
..............................................................................................................................................
(iii) By referring to its structure, explain why graphite flakes easily.
..............................................................................................................................................
..............................................................................................................................................
Q9.
(a) An experiment is carried out to measure the temperature change when solid ammonium chloride
is dissolved in water.
• initial temperature of water = 19°C
• final temperature of solution = 15°C
Explain what the temperature readings show about the type of heat change occurring when ammonium chloride dissolves in water.
(b) When zinc reacts with copper sulfate solution, copper and zinc sulfate solution are formed.
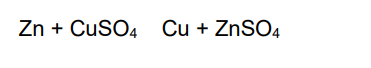
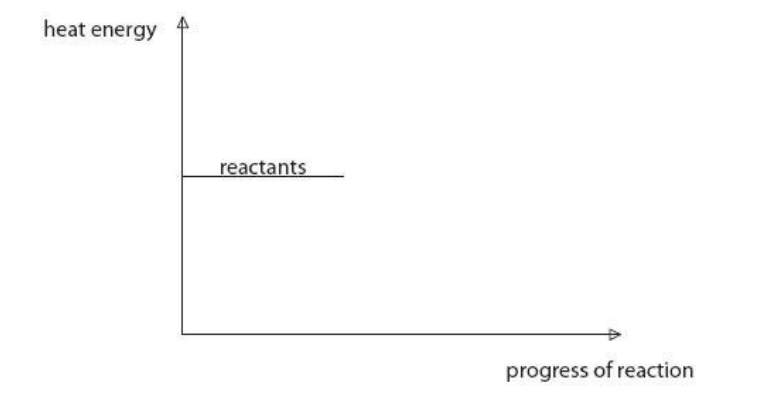
This reaction is exothermic.
Use this information to complete the diagram.
(c) Reactions are accompanied by heat changes.
The heat changes are the results of bonds being broken and bonds being formed.
Which row of the table shows the heat energy changes that occur when bonds are broken and when bonds are formed?

(d) Reactions can occur when particles collide.
Rates of reactions can be altered by changing conditions.
Explain how the rate of reaction between a solid and a liquid is altered by changing the size of the pieces of solid and by changing the temperature of the liquid.
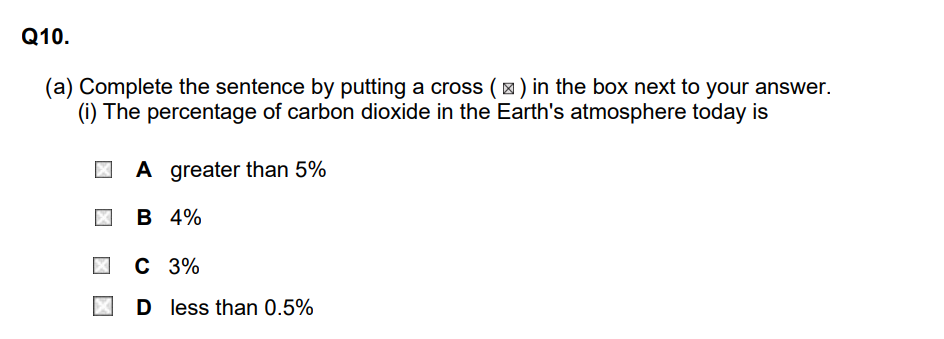
(ii) The percentage of carbon dioxide in the Earth's atmosphere today is less than that in the
Earth's earliest atmosphere.
Explain what has caused the percentage of carbon dioxide to decrease.
..............................................................................................................................................
(iii) Carbon dioxide and other gases in the atmosphere help to keep the Earth warm. State how these gases keep the Earth warm.
..............................................................................................................................................
(b) Describe the test to show that a gas is oxygen.
..............................................................................................................................................
..............................................................................................................................................
(c) Magnesium reacts with oxygen to form magnesium oxide.
An excess of magnesium ribbon was placed in an evaporating basin that was floated on water in a trough.
The magnesium ribbon was lit.
A bell jar was placed over the evaporating basin and the stopper inserted to seal the experiment.
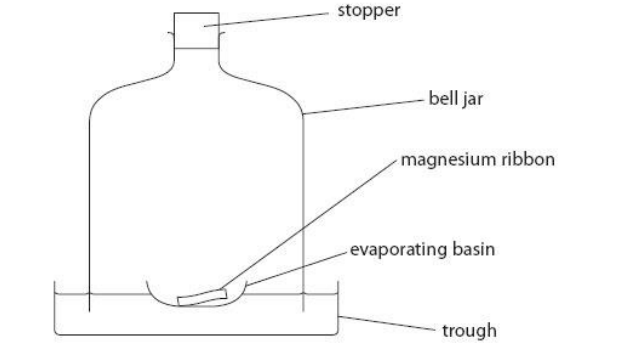
When the magnesium flame went out, there was some magnesium left in the basin.
When the apparatus had cooled, the water in the bell jar had risen.
(i) Explain why the water level had risen.
..............................................................................................................................................
..............................................................................................................................................
(ii) At the start of the experiment, the volume of the air in the bell jar was 1000 cm3.
Assume that 21% of the air by volume is oxygen.
Calculate the volume of gas that was present in the bell jar at the end of the experiment.
..............................................................................................................................................
..............................................................................................................................................
volume of gas = . . . . . . . . . . . . . . . . . . . . . . cm3
(d) Metal oxides react with acids to produce salts and water.
Dilute sulfuric acid was added to magnesium oxide.
State the name of the salt formed.
..............................................................................................................................................
Q11.
In industry sodium carbonate is made from sodium chloride solution and calcium carbonate in the Solvay Process.
(a) Describe the test to show that calcium carbonate contains carbonate ions.
..............................................................................................................................................
..............................................................................................................................................
..............................................................................................................................................
(b) Another product of the Solvay Process is calcium chloride.
Calculate the relative formula mass of calcium chloride, CaCl2.
(Relative atomic masses: Ca = 40; Cl = 35.5).
..............................................................................................................................................
(c) The overall equation for the Solvay Process is:
2NaCl + CaCO3 → Na2CO3 + CaC2
Calculate the maximum mass of sodium carbonate that could be formed by reacting 40 kg of calcium carbonate with an excess of sodium chloride solution. (Relative formula masses: CaCO3 = 100; Na2CO3 = 106).
..............................................................................................................................................
..............................................................................................................................................
(d) Sodium carbonate was made in a laboratory experiment.
The theoretical yield of the experiment was 15.0 g.
The actual yield of the experiment was 10.4 g.
(i) Calculate the percentage yield of sodium carbonate in this experiment.
..............................................................................................................................................
..............................................................................................................................................
(ii) Suggest two reasons why the actual yield was less than the theoretical yield.
..............................................................................................................................................
Q12.
(a) Two pieces of metal can be joined by welding them together.

(ii) To join two pieces of metal by welding, they must be melted together. State why a high temperature has to be used.
..............................................................................................................................................
(iii) The pieces of metal are welded together in an atmosphere of argon. Explain why an atmosphere of argon is used.
..............................................................................................................................................
(b) Some metals react with halogens.
Iron reacts with bromine, Br2, to form iron(III) bromide, FeBr3. Write the balanced equation for this reaction.
..............................................................................................................................................


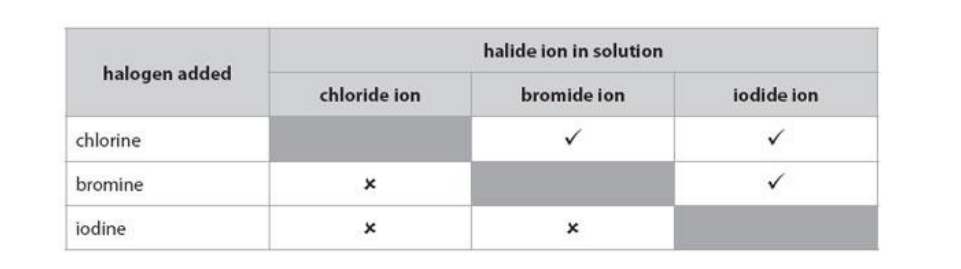
Use the information in the table to explain the order of reactivity of the three halogens.
..............................................................................................................................................
..............................................................................................................................................

Q14.
The diagram shows the electrolysis of hydrochloric acid.
Chlorine is produced in this process.
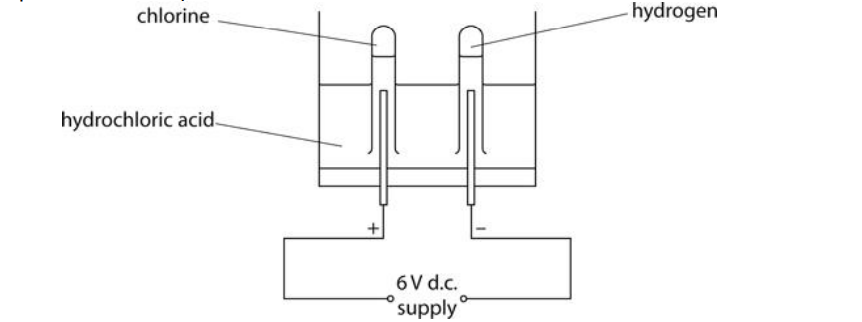
(i) Chlorine is given off in this experiment.
Describe the test to show the gas is chlorine.
..............................................................................................................................................
(ii) Explain one precaution that should be taken if hydrochloric acid is electrolysed on a large scale.
..............................................................................................................................................
以上是关于博耐顿女校Benenden School year12化学入学考试笔试题库的相关问题,总体来讲,低龄留学已成为了一种趋势,毕竟年龄越小,进入名校的机率越大。顶试留学是一所专注于英国低龄留学实行境内外一站式服务的机构,像留学选校、寻找寄宿家庭、寄宿生活、UKiset考试培训等提供全方位的留学咨询。在此,顶试留学预祝准留学生们都能学业有成!
想要获得已经就读该校的中国学生和家长关于学校的一手信息及评价?
扫码或长按添加微信
或者拨打电话进行咨询:
021-62110340
18217224528

点击“微信咨询”按钮
打开图示并自动复制微信ID“DSEdu1994”
打开微信,点击“添加朋友”,粘贴微信号并添加。